2 major types of gravimetric methods|thermal gravimetric method : exporter exporters exporting Gravimetry includes all analytical methods in which the analytical signal is a . Resultado da Fui criada, em 2005, com o objetivo de reunir acervos de livros em um único portal. Conecto leitores a pequenos livreiros, reúno exemplares novos e .
{plog:ftitle_list}
WEB10 de fev. de 2020 · Here’s a quick guide for watching Toronto Maple Leafs games with a .
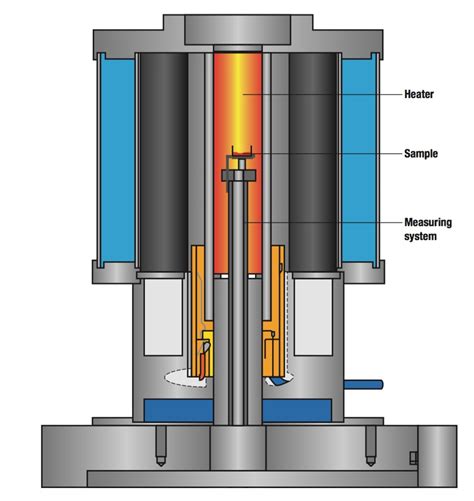
Types of Gravimetric Methods. The examples in the previous section illustrate four different ways in which a measurement of mass may serve as an analytical signal. When the signal is the mass of a precipitate, we call the method precipitation gravimetry.Example 8.4. The thermogram in Figure 8.10 shows the mass of a sample of .
thermal gravimetric method
Gravimetry includes all analytical methods in which the analytical signal is a .Types of Gravimetric Analysis. There are 4 fundamental types of gravimetric analysis. Of which, there are 2 common types involving changes in the phase of the analyte to separate it from the rest of a mixture, resulting in a change in . Gravimetry includes all analytical methods in which the analytical signal is a measurement of mass or a change in mass. When you step on a scale after exercising you .Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as the relative quantities of the other constituents are known.

gravimetric analysis, a method of quantitative chemical analysis in which the constituent sought is converted into a substance (of known composition) that can be separated .
There are different types of gravimetric analysis techniques, each with its unique approach and applications. Two common types include volatilization gravimetry and precipitation gravimetry. [7] Based on the separation of analyte, gravimetric analysis is of the following 4 types: Precipitation Gravimetry: The separation of one or more components of a solution by incorporating them into a solid is accomplished .1. Features of Gravimetric Analysis. A given analyte is isolated from the sample and weighed in some pure form. One of the most accurate and precise methods of macro quantitative .8A.2 Types of Gravimetric Methods The four examples in the previous section illustrate different ways in which the measurement of mass may serve as an analytical signal.
In contrast to volumetric analysis, which determines the amount of a desired element by measuring its volume, gravimetric analysis measures the amount of analyte by its mass. In volumetric analysis, the analyte’s volume is . Types of Gravimetric Methods. The examples in the previous section illustrate four different ways in which a measurement of mass may serve as an analytical signal. When the signal is the mass of a precipitate, we call the method precipitation gravimetry.
Contributors; Gravimetry includes all analytical methods in which the analytical signal is a measurement of mass or a change in mass. When you step on a scale after exercising you are making, in a sense, a gravimetric determination of your mass.If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Pre-laboratory Assignment: Gravimetric Analysis. Suppose that 0.323 g of an unknown sulfate salt is dissolved in 50 mL of water. The solution is acidified with 6 M \(\ce{HCl}\), heated, and an excess of aqueous \(\ce{BaCl2}\) is slowly added to the mixture resulting in the formation of a white precipitate.
7.5: Gravimetric Methods (Exercises) These are homework exercises to accompany "Chapter 8: Gravimetric Methods" from Harvey's "Analytical Chemistry 2.0" Textmap. 7.6: Gravimetric Methods (Summary) This is a summary to accompany "Chapter 8: Gravimetric Methods" from Harvey's "Analytical Chemistry 2.0" Textmap. A general principle of gravimetric method of analysis is based on a chemical reaction between analyte and reagent. The analyte (A) of molecules ‘a’ react with the reagent (R) of molecule ‘r’. After drying, the product formed by igniting AaRr can either be weighed or ignited to create another compound of known chemical components .12A-1 Properties of Precipitates and Precipitating Reagents A gravimetric precipitating agent should react specifically or at least selectively with the analyte and give precipitates that is: 1. Enough particle size for retaining on filter paper 2. High purity (free of contaminants) 3. Low solubility that no significant loss of the analyte occurs during filtration In most cases the precipitate is the product of a simple metathesis reaction between the analyte and the precipitant; however, any reaction that generates a precipitate potentially can serve as a gravimetric method. Most precipitation gravimetric methods were developed in the nineteenth century, or earlier, often for the analysis of ores .
This is a summary to accompany "Chapter 8: Gravimetric Methods" from Harvey's "Analytical Chemistry 2.0" Textmap. This is a summary to accompany "Chapter 8: Gravimetric Methods" from Harvey's "Analytical Chemistry 2.0" Textmap. . Method 4500-SO 4 2– C and Method 4500-SO 4 2 . Article type Section or Page License CC BY-NC-SA License .Gravimetric analysis is a key method for quantifying substances. It comes in two main flavors: precipitation and volatilization. Each type has its own strengths and is used for different kinds of samples. Choosing between precipitation and volatilization depends on the sample's properties.Note: basic descriptive statistics such as mean, median, and mode, as well as standard deviation, are not shown because most people are already familiar with them.In the diagram, they would fall under the “descriptive” analysis type. Tree Diagram Explained. The highest-level classification of data analysis is quantitative vs qualitative.Quantitative implies numbers while qualitative . There are two major types of gravimetric methods: Precipitation methods: in this method the analyte is converted to a sparingly soluble precipitate. This precipitate is then filtered, washed free of impurities, and converted to a product of known composition by suitable heat treatment, and the product is weighed.
The steps involved in the different gravimetry methods change slightly, and the process depends on the substance to be analyzed. The following are gravimetric analysis examples of each of the four .
There is extensive literature on RS, and its relationship to dietary fiber (Berry, 1986; Dai and Chau, 2017; Food Standards Australia New Zealand, 2017; Perera et al., 2010; Raigond et al., 2015) Enzymatic gravimetric methods for measuring fiber include RS to varying degrees depending on the type of RS, of which there are five classifications . There are two major types of gravimetric methods: Precipitation methods: in this method the analyte is converted to a sparingly soluble precipitate. This precipitate is then filtered, washed free of impurities, and .
There are two major types of gravimetric methods: Precipitation methods: in this method the analyte is converted to a sparingly soluble precipitate. This precipitate is then filtered, washed free of impurities, and . Because the release of a volatile species is an essential part of these methods, we classify them collectively as volatilization gravimetric methods of analysis. 8.4: Particulate Gravimetry Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participates in a chemical reaction.
how to calculate gravimetric temperature
356 Analytical Chemistry 2.0. 8A Overview of Gravimetric Methods. Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. Later, as you read through the de-scriptions of specific gravimetric methods, this survey will help you focus on their similarities instead of their differences.The four main types of this method of analysis are precipitation, volitilization, electro-analytical and miscellaneous physical method. The methods involve changing the phase of the analyte to separate it in its pure form from the original mixture and aPrecipitation method The precipitation method is the one used for the determination of the .The scale of operation and detection limit for particulate gravimetry can be extended beyond that of other gravimetric methods by increasing the size of the sample taken for analysis. This is usually impracticable for other gravimetric methods because of the difficulty of manipulating a larger sample through the individual steps of the analysis.
A storage method that gives both a high gravimetric energy density and a high volumetric energy density is, therefore, a requirement. . However, they are different from Type III in that the liner in Type III is mostly metal liner which contributes ≥5% of mechanical strength . The microspheres offer reasonable (5.4 wt%) gravimetric H 2 . Gravimetric Analysis. Gravimetric analysis is a quantitative method in analytical chemistry wherein the concentration of a substance present in a sample is evaluated based on the measurement of its mass. This is done by precipitating the analyte (substance being analyzed) in a sample as a solid compound which is then separated, washed, dried and weighed.

Types of Gravimetric Analysis : Precipitation Method : Precipitation Gravimetry splits one or more portions of a solution by integrating it into a strong solution using a precipitation reaction. Volatilization Method : Volatilization By heating or chemically decomposing the sample, we can separate components of our mixture using gravimetry.8A.2 Types of Gravimetric Methods The four examples in the previous section illustrate different ways in which the measurement of mass may serve as an analytical signal. When the signal is the mass of a precipitate, we call the method precipitation gravimetry. The indirect determination of PO 3 3– by precipitating Hg 2 Cl 2 is an ex-There are two types of gravimetric instruments: single-sided balance and double-sided balance. . 4.1.2 Gravimetric methods. . One of the main limitations of this technique is that a significant change in the scale growth process can occur as a result of intermediate interruptions. This is due to the thermal stresses arising during cooling .HA+BOH→BA+H 2 O. Acid + Alkali→Salt + Water. Or H + + A – + B + + OH – → B + + A – + H 2 O. Or H + + OH – → H 2 O. The acid-base titration is based on the reaction that neutralization is between a base or an acidic and analyte. In this type, a reagent is mixed with the sample solution until it reaches the required pH level.
What are the 2 major types of gravimetric methods? There are four fundamental types of gravimetric analysis: physical gravimetry, thermogravimetry, precipitative gravimetric analysis, and electrodeposition. These differ in the preparation of the sample before weighing of the analyte. Physical gravimetry is the most common type used in .
moisture meter for cactus
examples of gravimetric methods
Cifrado: Simplificada (guitarra y guitarra eléctrica) Cifrado Favorita. Tono: A. [Refrão] A. Tens liberdade aqui. Espírito de Deus, Espírito de Deus. E. Tens liberdade aqui.
2 major types of gravimetric methods|thermal gravimetric method